Scientists from the Tokai University School of Medicine discovered a class of chemical compounds with dual receptor-specific inhibitory activity.
Platelets cause blood to clot during an injury, preventing excessive blood loss. However, platelet aggregation can abnormally occur in inflammatory diseases or cancer. Treatments using the available antiplatelet agents can cause serious adverse effects, underscoring the need for more effective options. Accordingly, researchers discovered compounds derived from diphenyl-tetrazol-propanamides having novel dual-specific inhibitory activity. The compounds were able to target platelet aggregation induced by podoplanin and collagen, potentially finding applications as antiplatelet agents.
During injuries, such as cuts or other invasive damage to blood vessels, one of the primary responses of the human body is to activate platelets or thrombocytes to form a plug-like structure and prevent excessive bleeding. The platelets perform this plug-based repair role through a cascade of molecular signals often driven by structural proteins, such as collagen. Meanwhile, when malignant tumors that express podoplanin (PDPN) invade the blood vessels, they activate platelets. However, blood clots can accumulate to an extent to obstruct a blood vessel, such as an artery or a vein, resulting in a condition known as thrombosis. If not managed correctly, thrombosis can lead to organ failure and other related complications.
Conventionally used antiplatelet therapeutic agents are associated with adverse effects, such as increased risk of bleeding. Therefore, developing targeted antiplatelet agents is the need of the hour. Antiplatelet agents that target specific receptors and thereby interfere with blood clot or thrombus formation are needed.
The platelet C-type lectin-like receptor 2 (CLEC-2) can be clustered by PDPN binding. This mechanism leads to platelet activation and aggregation, resulting in thrombus formation. Notably, this mechanism could result in life-threatening conditions, such as venous thromboembolism and tumor metastasis. Small-molecule compounds that interfere with the PDPN–CLEC-2 axis could be effective antiplatelet agents.
Accordingly, a team of scientists from Japan comprising Dr. Nobuo Watanabe, Assistant Professor at the Department of Emergency and Critical Care Medicine from Tokai University School of Medicine, Japan, along with Dr. Noriaki Hirayama from The Institute of Medical Sciences from Tokai University, and Dr. Sadaki Inokuchi from Tokai University School of Medicine have recently discovered a class of compounds that inhibit the platelet activation mechanism. The study was published online on 15 November 2023 in the journal Thrombosis and Haemostasis.
When asked about the motivation behind this study, Dr. Watanabe says, “From our previous research, we discovered and understood the role of PDPN in activating platelets, which crucially corresponded to poor cancer prognosis. These findings encouraged our team to develop novel antiplatelet agents targeting the interaction between PDPN and CLEC-2, which is a transmembrane receptor on the surface of platelets.”
The researchers initially identified a distinct class of chemical compounds containing a diphenyl-tetrazol-propanamide structure. They achieved this feat through computer-based molecular docking studies on over 7,000,000 compounds. The hit compounds were then analyzed using a PDPN–CLEC-2 binding inhibition assay. The researchers found that twelve such compounds inhibited the platelet aggregation induced by PDPN.
The researchers also discovered that their hit compounds interacted strongly with the glycoprotein VI (GPVI) protein molecule, inhibiting the collagen-induced platelet aggregation. These results indicated that the compounds had dual-specific inhibitory activity.
“It is acknowledged among platelet researchers that discovering a small-molecule compound that can inhibit the binding of CLEC-2-PDPN or GPVI-collagen is extremely challenging. Our research team has discovered that the diphenyl-tetrazol-propanamide chemical structure is fundamental to the inhibitory activity against both platelet receptors. We hope our novel findings can find use in academia or the pharmaceutical industry to develop new antiplatelet agents against life-threatening disorders such as arterial and venous thrombosis”, concludes Dr. Watanabe.
Thanks to the efforts of the researchers, the novel class of antiplatelet agents derived from the diphenyl-tetrazol-propanamide chemical structure may significantly improve the treatment outcomes and reduce the death rates associated with thrombosis.
Reference
Authors
Nobuo Watanabe1,2, Yoshiko Shinozaki3, Sanae Ogiwara3, Riko Miyagasako3, Ayumi Sasaki3, Junko Kato3, Yusuke Suzuki3, Natsuko Fukunishi3, Yoshinori Okada3, Takeshi Saito1, Yumi Iida3, Misaki Higashiseto3, Haruchika Masuda4, Eiichiro Nagata5, Kazuhito Gotoh6, Mari Amino1, Tomoatsu Tsuji1, Seiji Morita1, Yoshihide Nakagawa1, Noriaki Hirayama2,7, and Sadaki Inokuchi1,2
Title of original paper
Diphenyl-tetrazol-propanamide Derivatives Act as Dual-Specific Antagonists of Platelet CLEC-2 and Glycoprotein VI
Journal
Thrombosis and Haemostasis
Affiliations
1 Department of Emergency and Critical Care Medicine, Tokai University School of Medicine, Japan
2 Institute of Advanced Biosciences, Tokai University, Japan
3 Support Center for Medical Research and Education, Tokai University, Japan
4 Department of Physiology, Tokai University School of Medicine, Japan
5 Department of Neurology, Tokai University School of Medicine, Japan
6 Department of Laboratory Medicine, Tokai University School of Medicine, Japan
7 The Institute of Medical Sciences, Tokai University, Japan
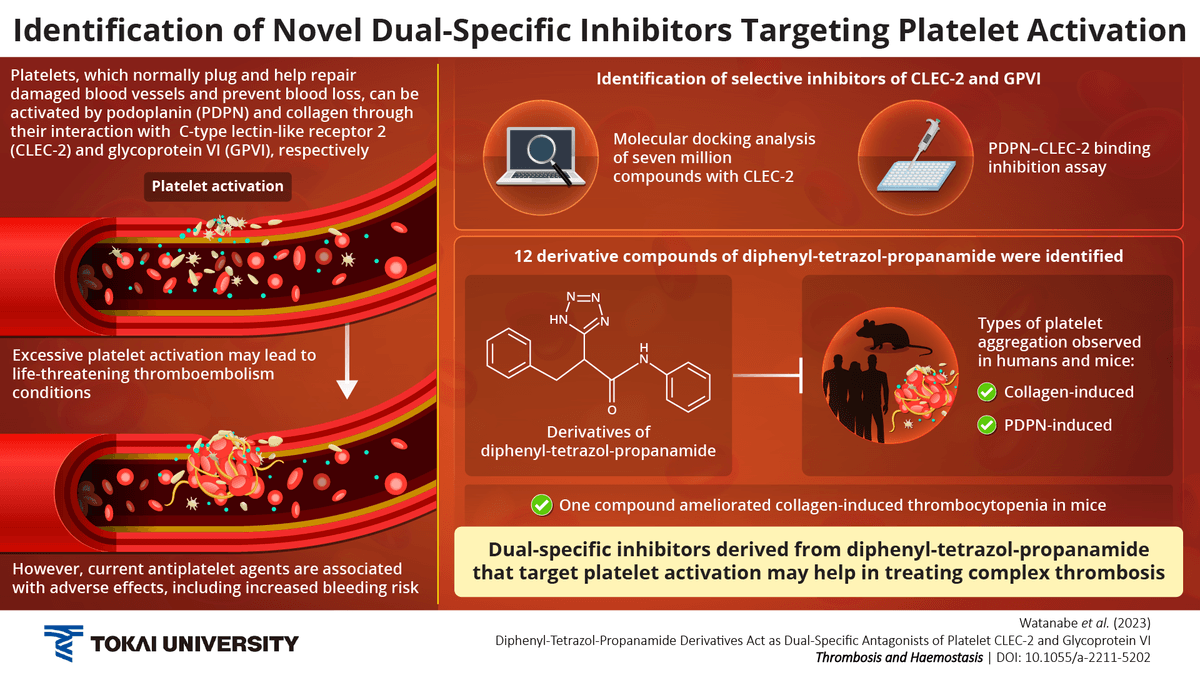
Image title: Dual receptor-specific antiplatelet agents for treating abnormal platelet aggregation
Image caption: Diphenyl-tetrazol-propenamide-based derivative compounds, such as compound G14 developed by Tokai University scientists, are promising dual-specific antiplatelet agents. These compounds can selectively inhibit podoplanin and collagen-induced platelet aggregation.
Image credit: Dr. Nobuo Watanabe from Tokai University School of Medicine, Japan
License: Original content
Usage restrictions: Not to be reused without permission
Additional information for EurekAlert
Latest Article Publication Date:
Method of Research:
Subject of Research:
Conflicts of Interest Statement:
29 December 2023
Experimental study
Animals
The authors declare no conflicts of interest.
About Assistant Professor Nobuo Watanabe
Nobuo Watanabe is an assistant professor at the Department of Emergency and Critical Care Medicine, Tokai University School of Medicine, Japan. He has completed his higher studies at Tokyo University of Science, Japan. He has trained and worked at the University of Alabama at Birmingham (UAB), USA, for a total of 5 years. He joined Tokai University School of Medicine as a faculty in 2013. His primary areas of interest include oxidative stress biomarkers, inflammatory responses, sepsis, and cancer. He has secured competitive grants for multiple research areas, such as sepsis management and alternatives to physical therapy. He has published over 40 research papers that have already received more than 1150 citations.
Media contact
Dr. Nobuo Watanabe
Assistant Professor
Department of Emergency and Critical Care Medicine
Tokai University School of Medicine, Japan
Email ID: wn600365@tsc.u-tokai.ac.jp